Cerezyme is the ONLY ERT (enzyme replacement therapy) that has shown long-term efficacy and safety in multiple studies over 20 years and has been prescribed for over 25 years.
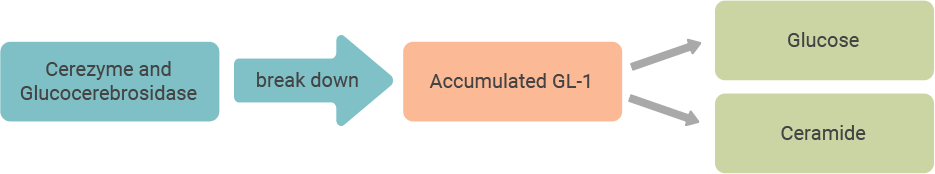
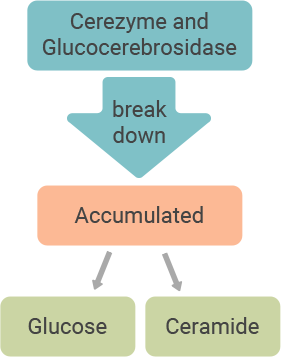
People with Gaucher disease type 1 may not have enough of an enzyme called acid beta-glucocerebrosidase (pronounced gloo-ko-ser-e-bro-sydaze) that breaks down a fatty substance called glucosylceramide (gloo-ko-sil-sara-mide), or GL-1. This may cause a build-up of GL-1 in key organs such as the spleen and liver, as well as in the bones, leading to the signs and symptoms of Gaucher disease type 1.
Cerezyme is a modified form of the enzyme glucocerebrosidase, and it works to reduce the build-up of GL-1 in the body.
Cerezyme has been used since 1994 in thousands of patients with Gaucher disease type 1 around the world
Cerezyme is designed to reduce the build-up of GL-1 in people who do not have enough of the natural enzyme to keep up with their bodies’ production.
Cerezyme acts like glucocerebrosidase (the
body’s natural enzyme), breaking down GL-1 into its more basic elements, glucose and ceramide, that can be naturally removed from the body.
The most studied ERT in children and adults with Gaucher disease type 1
See resultsLearn the causes and symptoms of Gaucher disease type 1
Get factsCerezyme® (imiglucerase) for injection is indicated for treatment of adults and pediatric patients 2 years of age and older with Type 1 Gaucher disease that results in one or more of the following conditions:
Hypersensitivity and Infusion-Associated Reactions: Serious allergic reactions (anaphylaxis) have been reported in patients treated with Cerezyme. Symptoms suggestive of an allergic reaction have been reported during or shortly after an infusion and include itching, flushing, hives, swelling, chest discomfort, shortness of breath, coughing, cyanosis (a bluish discoloration of the skin due to diminished oxygen), rapid heart rate, and low blood pressure. Inform your doctor and seek medical care if you experience any of these symptoms. If you have had an allergic reaction to Cerezyme, you and your doctor should use caution if you continue to receive treatment with Cerezyme.
Immune Responses: Approximately 15% of patients have developed immune responses (antibodies) to Cerezyme during the first year of therapy. These patients have a higher risk of an allergic reaction (hypersensitivity). Your doctor may periodically test for the presence of antibodies.
Adverse Reactions:
Adverse reactions reported in adults include back pain, chills, dizziness, fatigue, headache, hypersensitivity reactions, nausea, pyrexia, and vomiting.
Adverse reactions reported in pediatric patients 2 years of age and older are similar to adults.
Please see the Full Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact Sanofi Medical Information at 1-800-745-4447, Option 2.