Bone Studies
Cerezyme is the ONLY ERT (enzyme replacement therapy) that has
shown long-term efficacy and safety in multiple studies over 20 years
and has been prescribed for over 25 years.1-3
Cerezyme: effect on bone mineral density (BMD), bone pain, and bone crises4-6
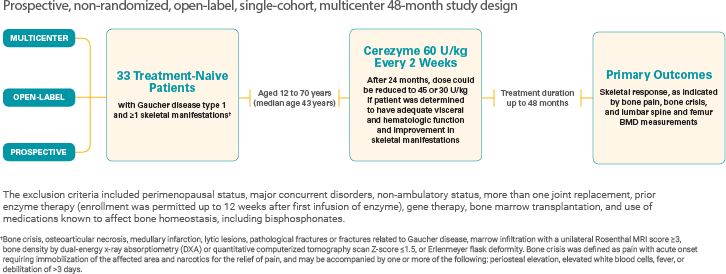
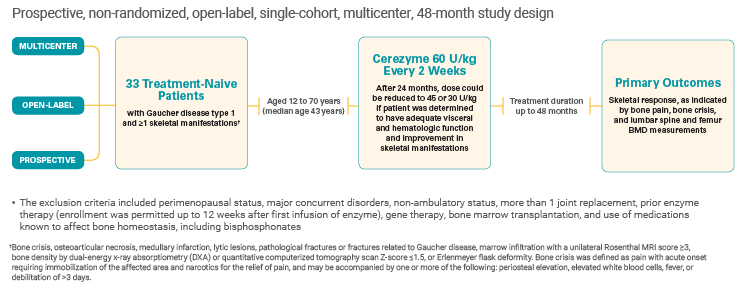
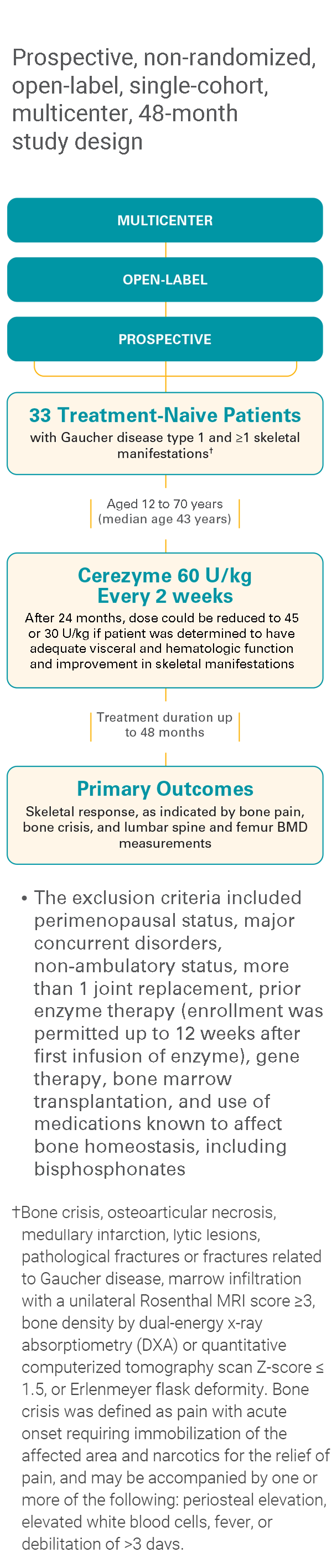
An overview: Cerezyme studies for bone manifestations in Gaucher disease type 1
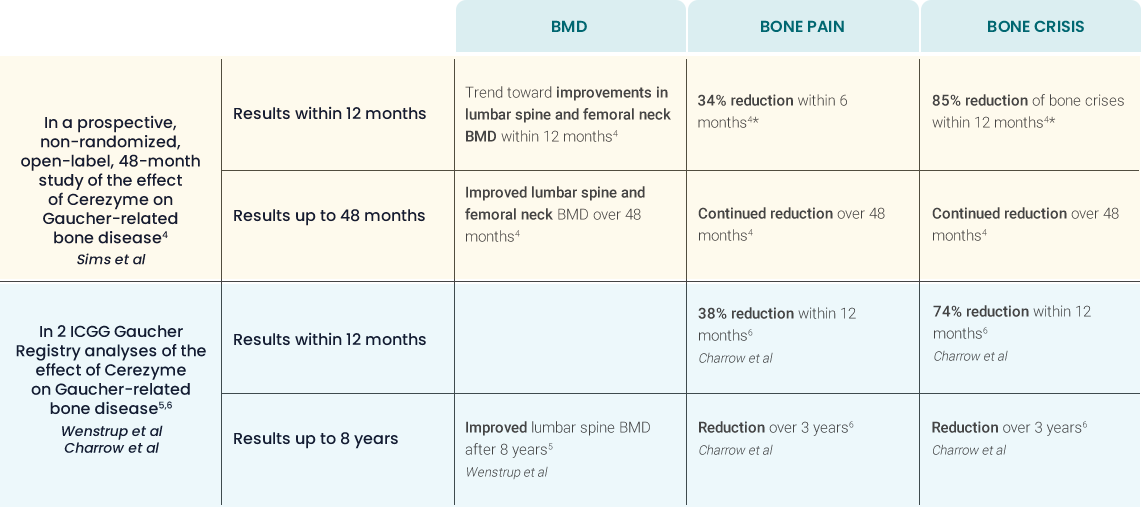
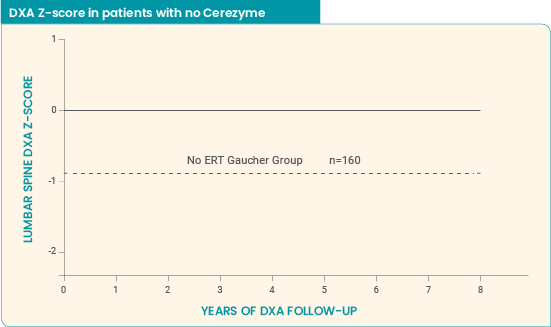
Long-term effect of Cerezyme on BMD4
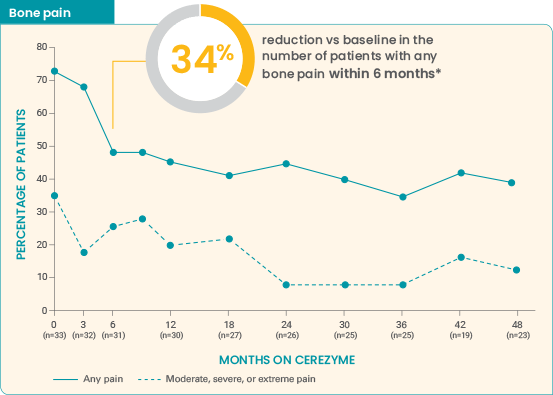
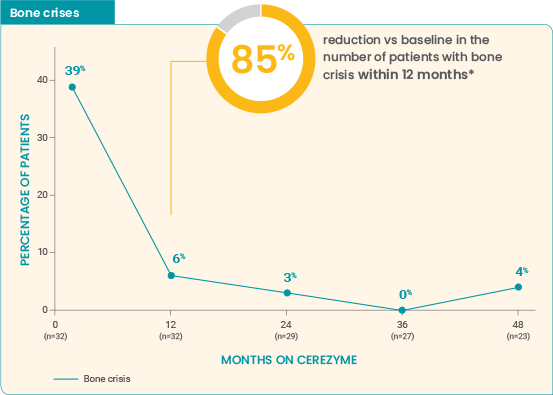
Long-term effect of Cerezyme on the occurrence of
bone pain and bone
crises4
Safety from Sims et al4
- The most common AEs were chills, flushing, and arthralgia, each reported in 4 patients (12%)
- One patient withdrew from the study because of a severe infusion reaction, including anxiety, chest pain, hypertonia, chills, tachycardia, and vomiting, from which he recovered without sequelae
AE=adverse event; BMD=bone mineral density; DXA=dual-energy x-ray absorbtiometry.
Retrospective ICGG Gaucher Registry studies
8-year ICGG Gaucher Registry analysis (Wenstrup et al): Study parameters5
An observational, retrospective analysis in adults (men aged 18 to 70 years, women aged 18 to 50 years) enrolled in the ICGG Gaucher Registry for whom lumbar spine BMD measurements were available. Read more...
Cerezyme impact on BMD over 8 years5
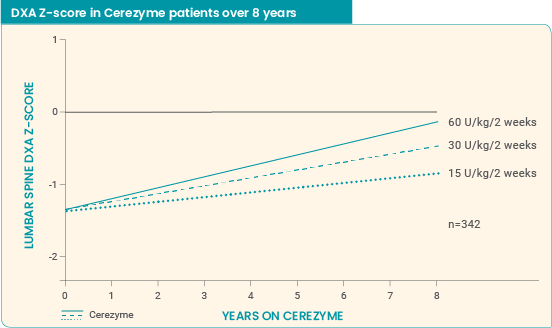
- Cerezyme dosing should be individualized to each patient1
- The recommended dosage of Cerezyme based upon disease severity ranges from 2.5 U/kg 3 times a week to 60 U/kg once every 2 weeks1
- Titrate the dosage based on clinical manifestations of disease and therapeutic goals for the patient1
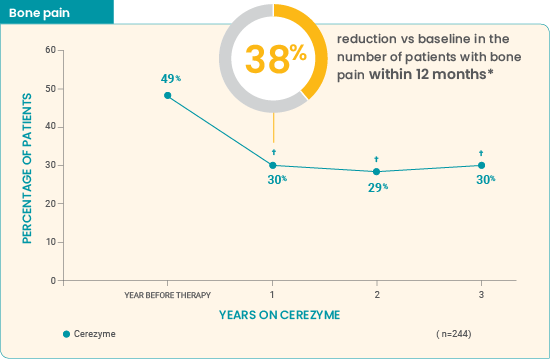
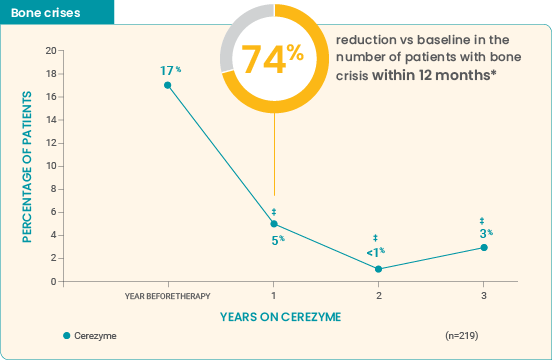
4-year ICGG Gaucher Registry analysis (Charrow et al): Study parameters6
This retrospective analysis used data from the ICGG Gaucher Registry on patients with bone crisis and/or bone pain data for 1 year prior to Cerezyme, and each year for 3 years after the start of Cerezyme. The year before treatment was considered to be baseline. Data on bisphophonate use were not available. Read more...
Effect of Cerezyme for injection on the occurrence of bone pain and bone crises over 4 years6
Efficacy in key disease parameters in pediatric patients with Gaucher disease type 1
Explore pediatric data