8-Year Pediatric Study
Choose Cerezyme: an established ERT for children ages 2 and up1
Choose Cerezyme: an established ERT for children ages 2 and up1,7
The safety and effectiveness of Cerezyme have been established in pediatric patients 2 years of age and older1:
- Evidence from adequate and well-controlled studies of Cerezyme and alglucerase in adults and pediatric patients 12 years of age and older
- Additional data obtained from the medical literature and from postmarketing experience in pediatric patients as young as 2 years of age
- The safety and effectiveness in patients younger than 2 years have not been established
Cerezyme* improved long-term visceral and hematologic manifestations in pediatric patients in an 8-year ICGG Gaucher Registry study7
Study description (Andersson et al)7
This observational study used data derived from the International Collaborative Gaucher Group (ICGG) Gaucher Registry submitted between 1991 and January 2006. Data were retrospectively analyzed for all patients (n=884) with Gaucher disease type 1 who had intact spleens and were receiving alglucerase (22.4%) or Cerezyme (77.6%).Read more...
Studied in the largest reported group of treated pediatric patients (n=884)
with Gaucher disease type 1 around the world7
Cerezyme* reduced spleen and liver volumes in children7
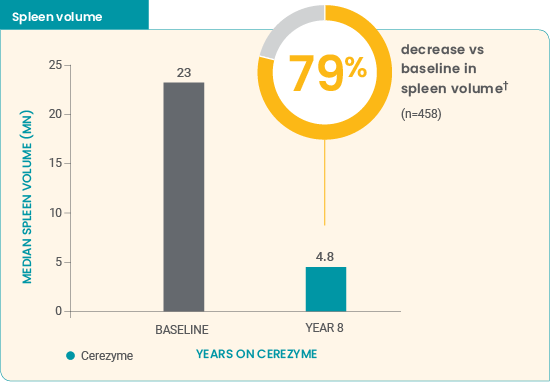
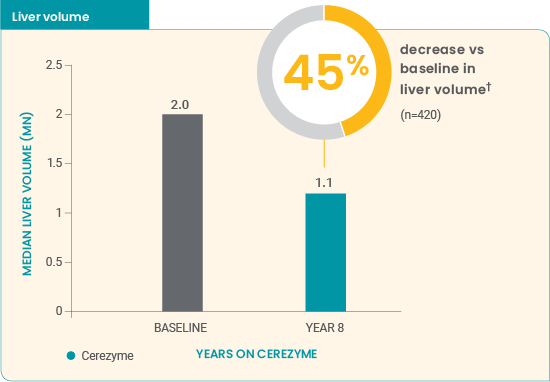
- Approximately half of the treatment effect was achieved after ≈1 year of treatment
- Volume reductions continued over the entire 8-year study period
Improvements were seen in children treated with Cerezyme* over 8 years
Cerezyme* improved hematologic parameters in children7
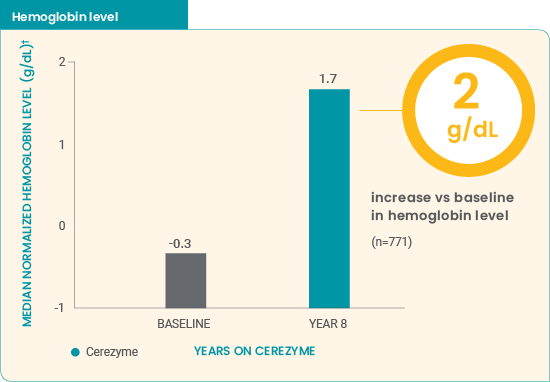
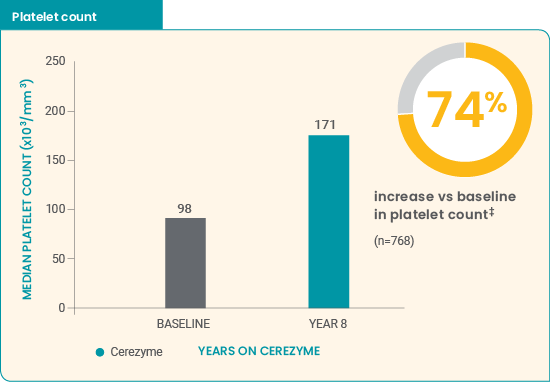
- Improvements in median normalized hemoglobin level were demonstrated during the first year of treatment
- Median hematologic parameters increased to levels similar to those in the normal population after 8 years
0% of children on Cerezyme had anemia after 6 years
Cerezyme* effect on bone mineral density (BMD) over 12 years in children7
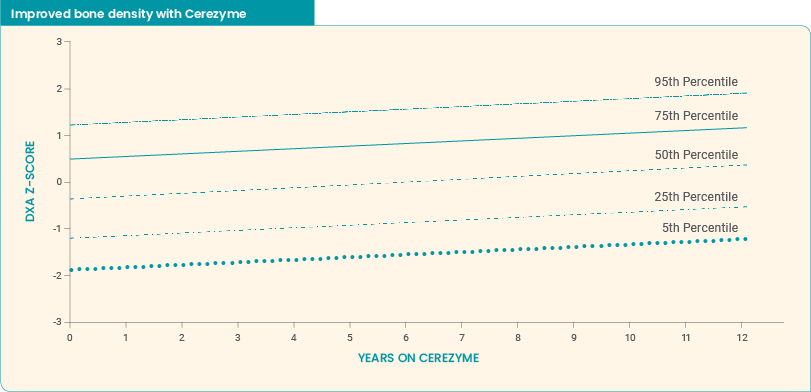
The DXA Z-scores for patients in the 95th, 75th, 50th, 25th, and 5th percentiles at first infusion were 1.22, 0.49, -0.35, -1.19, and -1.93, respectively. At 12 years, the DXA Z-scores for patients in the 95th, 75th, 50th, 25th, and 5th percentiles were 1.87, 1.13, 0.29, -0.55, and -1.29. N=127; BMD Z-score intercepts and slopes (change over time) were monitored for 12 years.
Timing of pediatric treatment should consider that most bone mineral is accrued in the first 2 decades of life and BMD peaks in the third decade

