6-Month Pivotal Study
Cerezyme is the ONLY ERT (enzyme replacement therapy) that has
shown long-term efficacy and safety in multiple studies over
20 years
and has been prescribed for over 25 years.1-3
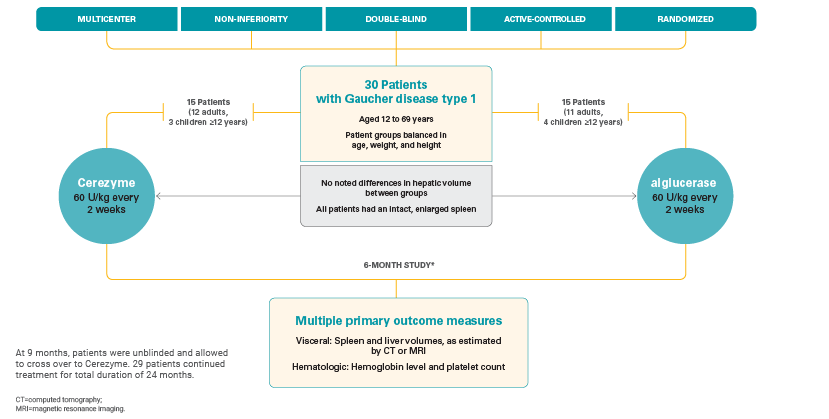
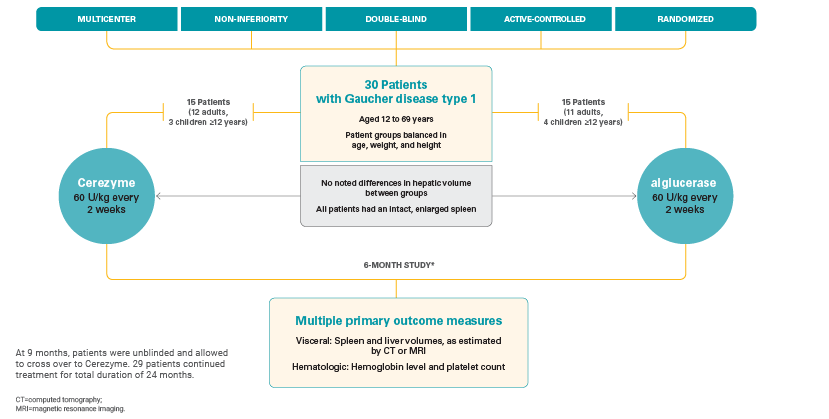
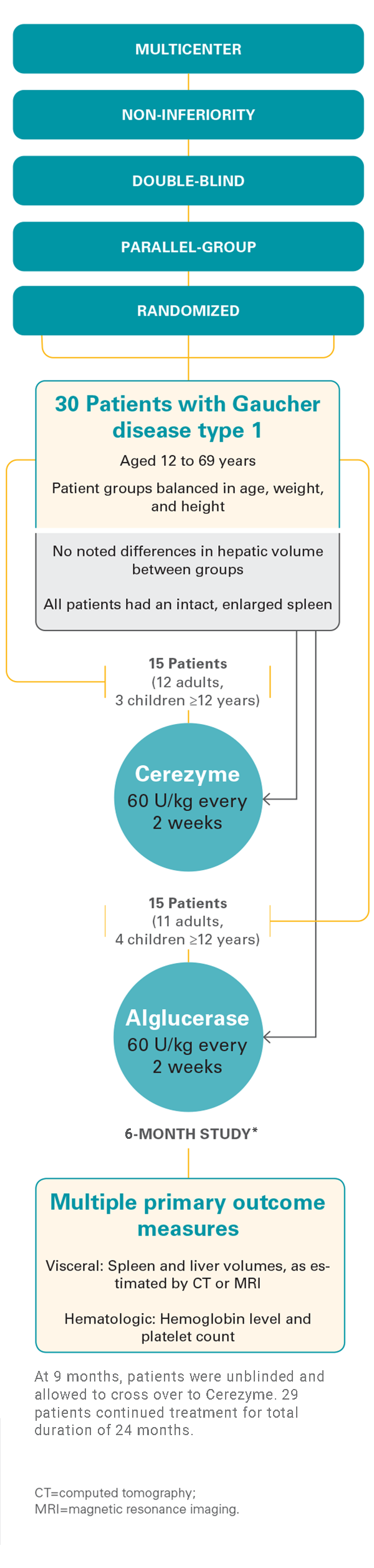
Pivotal, phase 3 study
Cerezyme was proven to be safe and effective, as shown in a pivotal, phase 3 study that studied visceral, hematologic, and bone parameters in adult and pediatric patients with Gaucher disease type 1.1
Study design1
Cerezyme showed similar reductions in
visceral parameters at 6 months vs alglucerase1
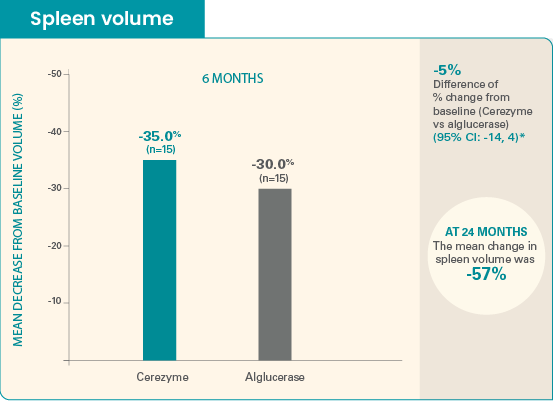
The mean baseline spleen volume was 2369 mL for Cerezyme patients and 2603 mL for alglucerase patients.
The absolute change from baseline was -902 mL for Cerezyme and -874 mL for alglucerase. Difference of absolute change was -28 mL (95% CI: -652, 596).
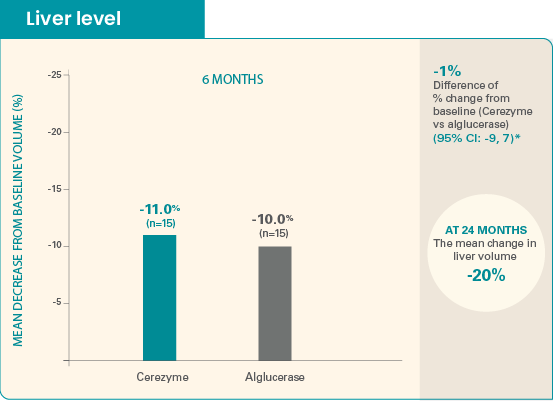
The mean baseline liver volume was 2521 mL for Cerezyme patients and
2788 mL for alglucerase
patients.
The absolute change from baseline was -310 mL for Cerezyme and -307 mL for alglucerase. Difference of absolute change was -3 mL (95% CI: -246, 240).
Cerezyme showed similar improvements in
hematologic parameters at 6 months vs alglucerase1
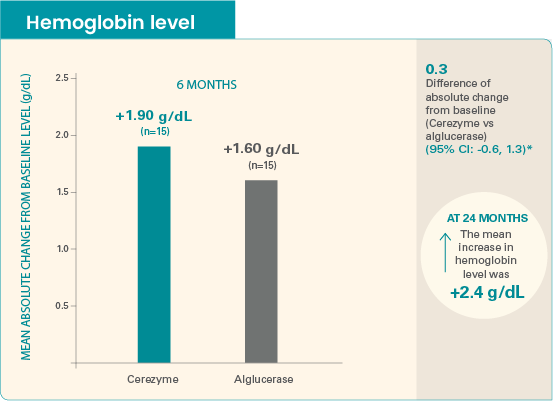
The mean baseline hemoglobin level was 10.7 g/dL for Cerezyme patients and 10.9 g/dL for alglucerase patients.

The mean baseline platelet count level was 68.5 x 103/mL3 for Cerezyme patients and 74.2 x 103/mL3 for alglucerase patients.
Bone x-rays showed improvements in cortical thickness and lucencies
in 7 of 11 Cerezyme-treated patients

Statistically significant improvements evaluated over 20 years
See results